Strong Acids Vs Weak Acids
Strong Acids Vs Weak Acids. Difference between strong and weak acids. The opposite if true for the weak base.
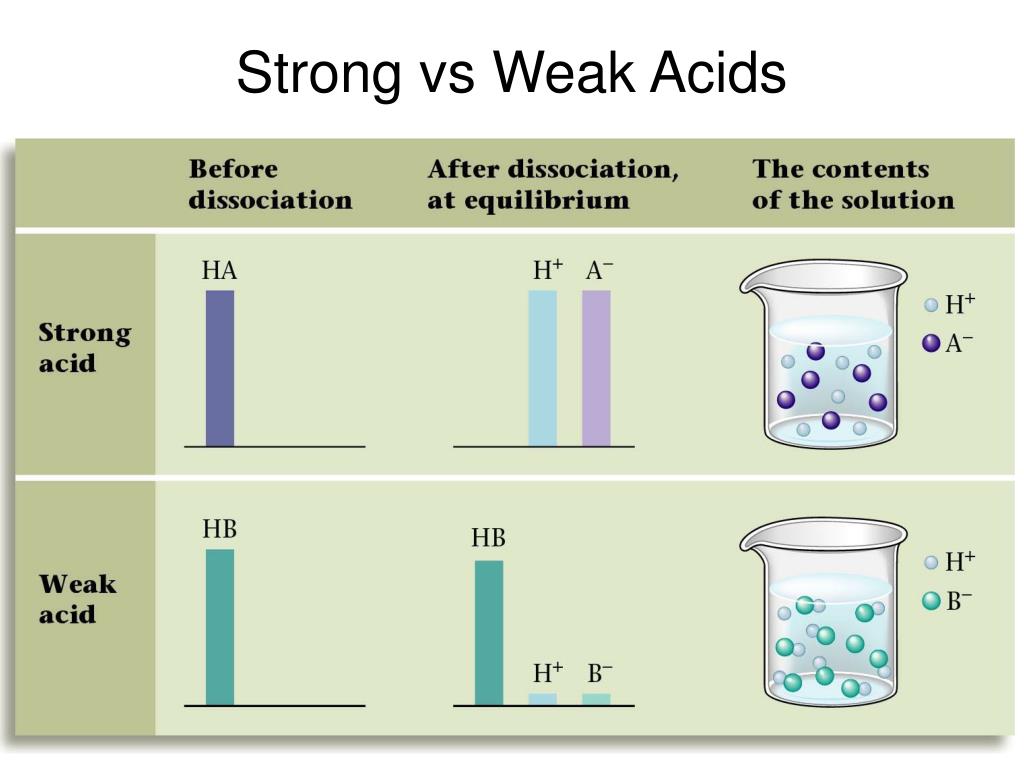
 PPT Ionization Constants of Acids and Bases Strengths from www.slideserve.com
PPT Ionization Constants of Acids and Bases Strengths from www.slideserve.comIn contrast, a strong acid fully dissociates into its ions in water. The opposite if true for the weak base. The ions react very easily to reform the acid and the water.
![Strong and Weak Acids and Bases [Video] in 2020](https://i.pinimg.com/736x/3f/df/36/3fdf3642f87abd7de476e11d8ae41841.jpg)
Ethanoic acid is a typical weak acid. The opposite if true for the weak base.
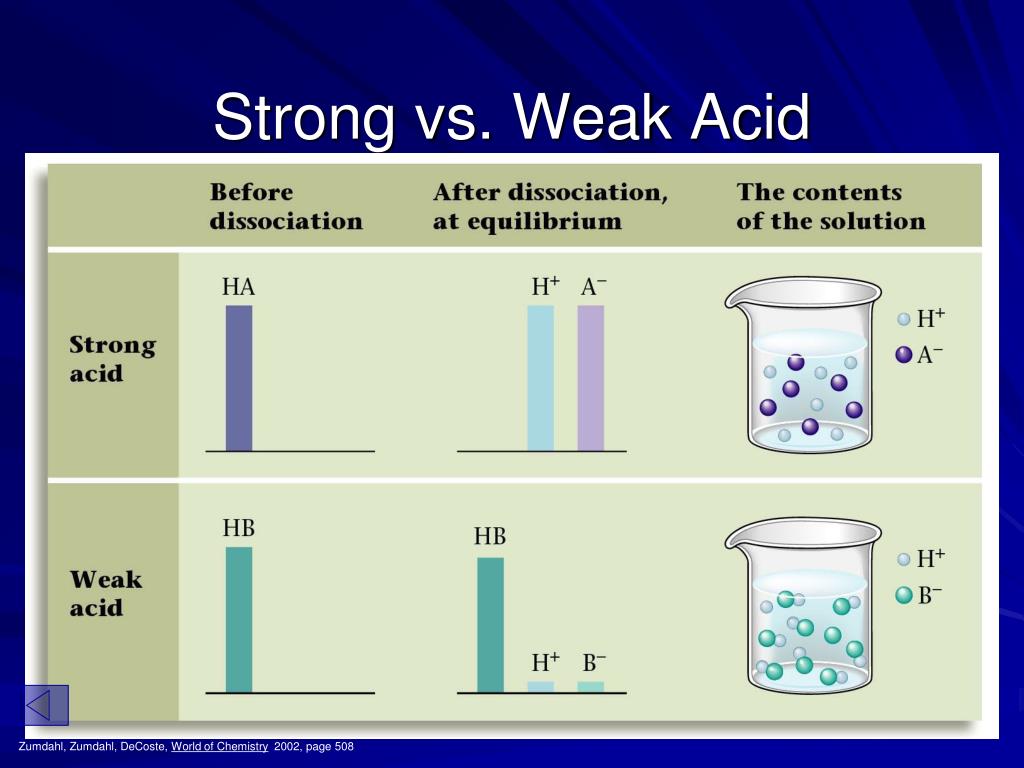
Amino acids without pictures 1. Rate of reaction is faster.

Therefore, a strong acid will completely disassociate to form hydrogen ions while a weaker acid will only partially disassociate. Hcl, hbr, hi, hno 3, hclo 4, h 2 so 4 the following are some less common acids that are also strong:
Weak acids ionize partially in an. It is a poor proton donor.

Difference between strong and weak acids definition. Most molecules of strong acid split into ions water and produce large amounts of hydrogen ions.

It reacts with water to produce hydroxonium ions and ethanoate ions, but the back reaction is more successful than the forward one. When chemists refer to strong and weak acids they are referring to the degree with which the acid molecules break apart to give ions in aqueous solution (dissociation).
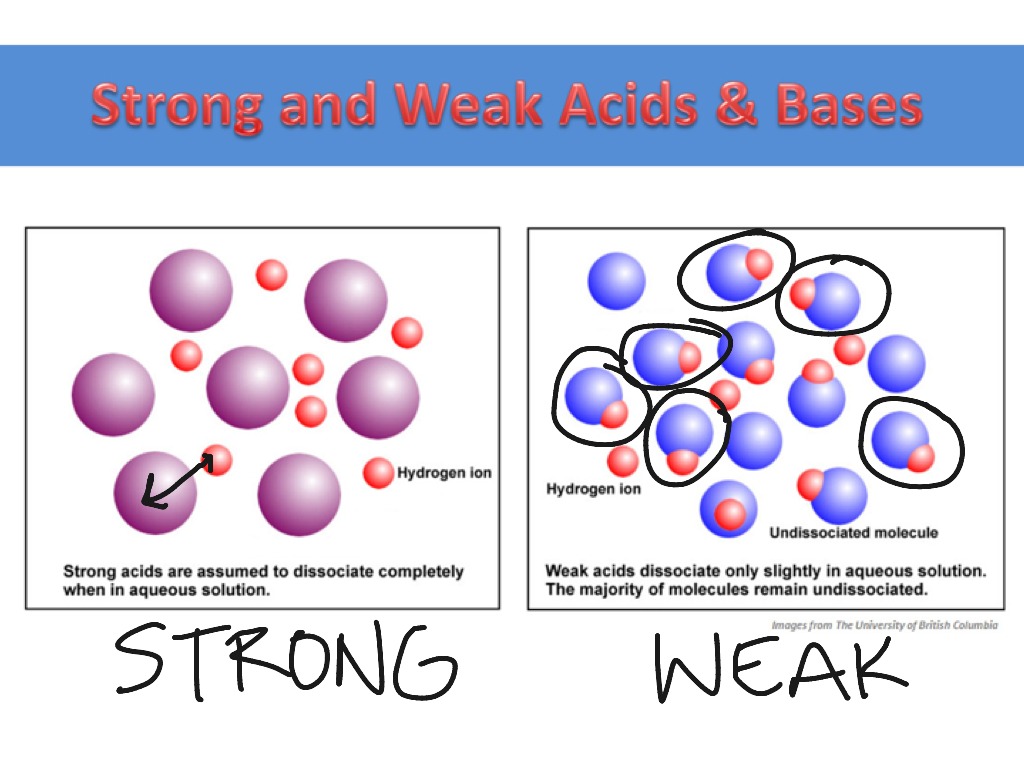
Strong acid add all their h+ to will weak acid only add some h+ to solution. The primary difference between strong and weak acids is how the behave in water.
One of the most confusing points with acids is the difference between strength and concentration. Therefore, a strong acid will completely disassociate to form hydrogen ions while a weaker acid will only partially disassociate.
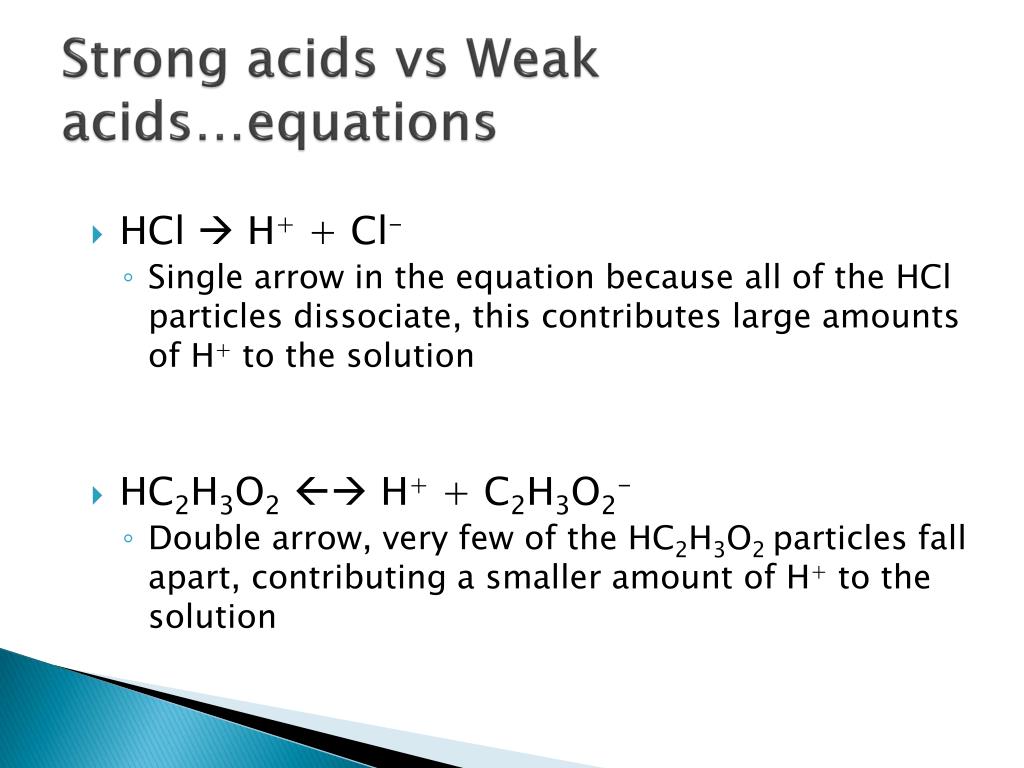
Strong acids react faster whereas weak acids take time to react with any base. Similarly one may ask, what is the difference.

Strong acids are molecules that completely dissociate into their ions when it is in water. Difference between strong and weak acids.

The opposite if true for the weak base. Strong acid passes electricity faster whereas weak acids are slow conductors.
Ethanoic acid is a typical weak acid. The same thing goes for bases and alkali:
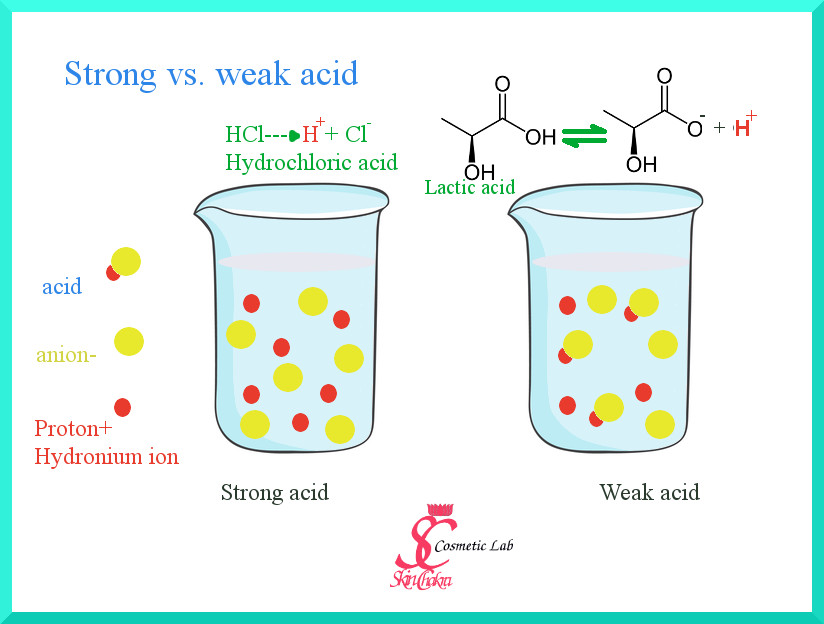
A weak acid is an acid that partially dissociates into its ions in an aqueous solution or water. An acid or a base is strong when they dissociate completely in an aqueous medium, that is, the ionization process is complete and the solution will contain the same concentration of.
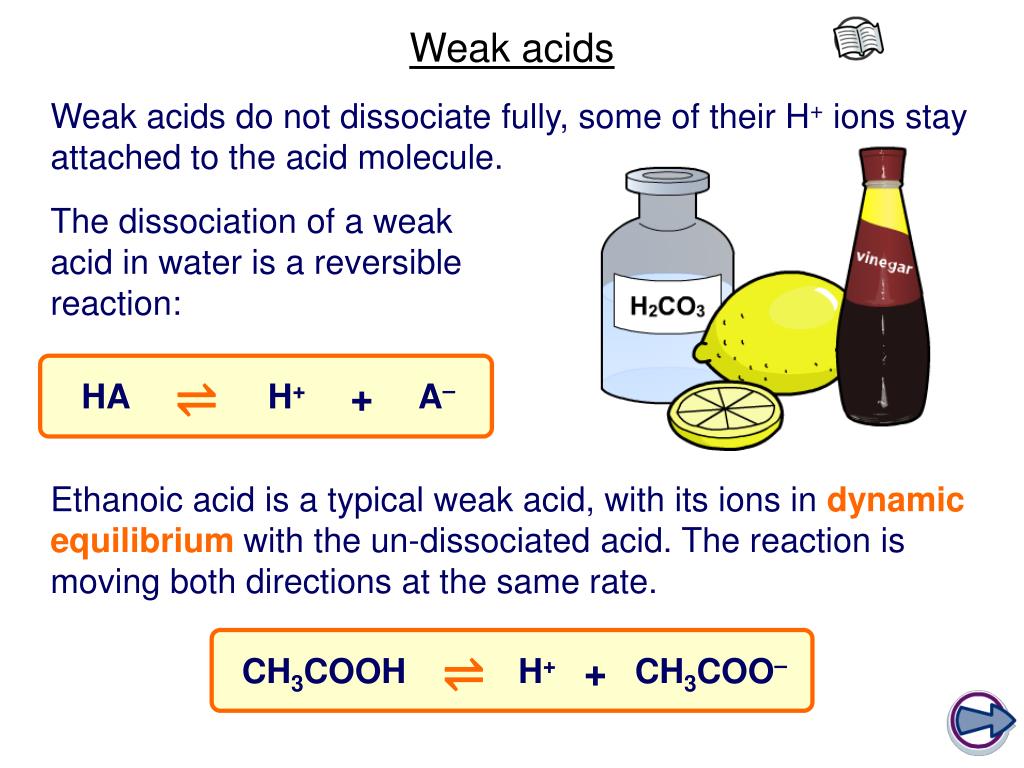
Therefore, a strong acid will completely disassociate to form hydrogen ions while a weaker acid will only partially disassociate. Strong acid passes electricity faster whereas weak acids are slow conductors.
/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)
The dissociation of a weak acid is a reversible reaction that reaches equilibrium. Strong acids are molecules that completely dissociate into their ions when it is in water.
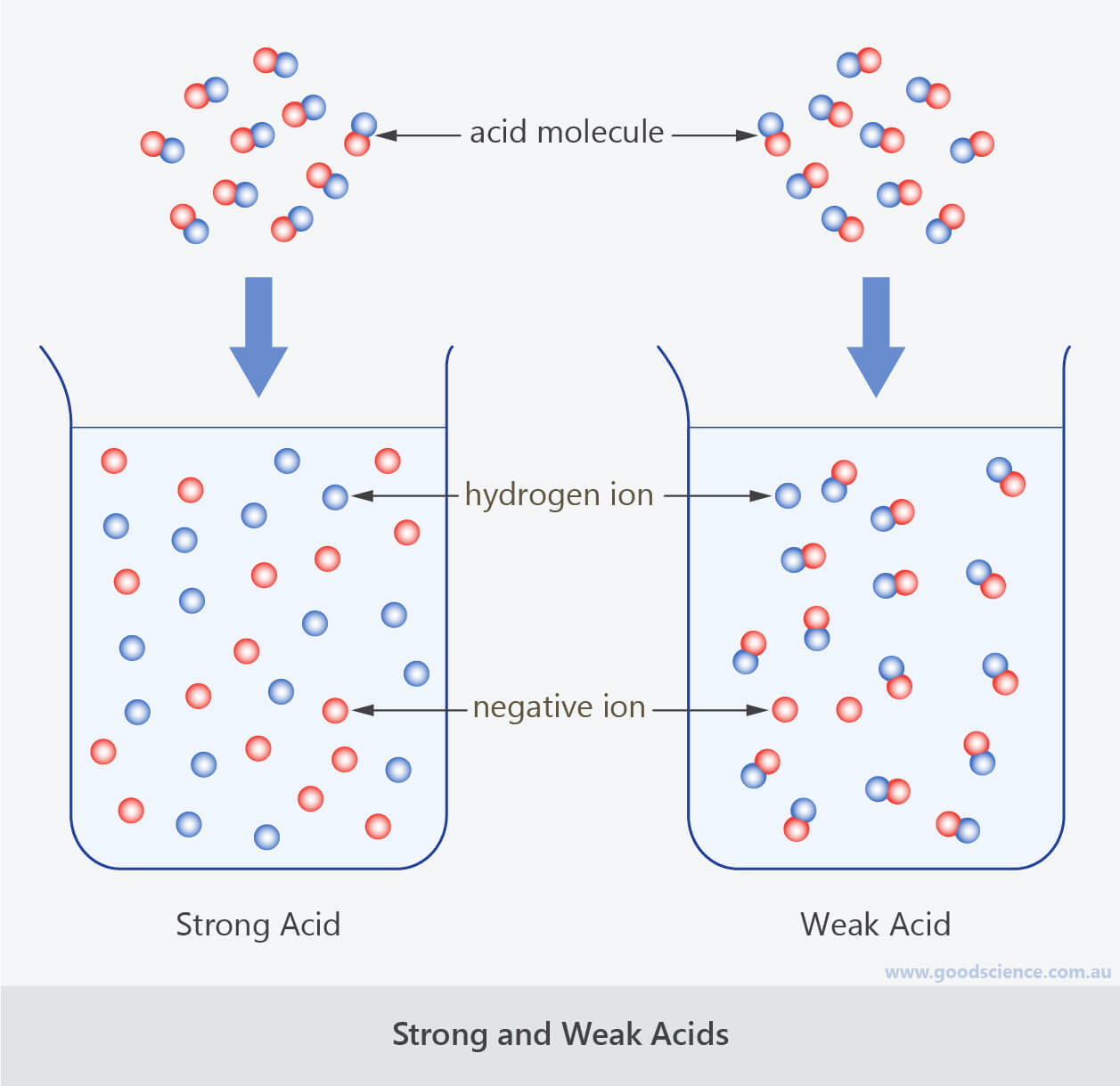
Difference between strong and weak acids. Hf, hno 2, hclo 2, [h 2 so 3] = so 2 + h 2 o, hc 2 h 3 o 2 = hoac 2.
Strong acids ionize completely in an aqueous solution. The concentration of an acid is the number of mols of that acid per liter of water.
/list-of-strong-and-weak-acids-603642-v2copy2-5b47abd0c9e77c001a395e55.png)
It is a poor proton donor. A weak acid has a higher buffer capacity for a strong base.
Hf, hno 2, hclo 2, [h 2 so 3] = so 2 + h 2 o, hc 2 h 3 o 2 = hoac 2. Difference between strong and weak acids.

It always loses h +, when dissolved in water. Rate of reaction is faster.
The Opposite If True For The Weak Base.Weak acids are molecules that partially dissociate into ions in aqueous solution. These react less violently with metals to give. It possess high conductivity due to the presence of the unpaired atoms.
A Strong Acid Or Base Is One That Is Completely Ionized In A Solution.Phenolphthalein is usually preferred because of its more easily seen colour change. Difference between strong and weak acid acids are chemical substances that donate hydrogen ions or protons when mixed in solutions. Hf, hno 2, hclo 2, [h 2 so 3] = so 2 + h 2 o, hc 2 h 3 o 2 = hoac 2.
Main Differences Between Strong Acid And Weak Acid.Identifying weak acids and strong acids (practice) | khan academy. The same thing goes for bases and alkali: It always loses h +, when dissolved in water.
Click To See Full Answer.Difference between strong and weak acids. The conjugate base of a weak acid is a weak base, while the conjugate acid of a weak base is a weak acid. This means that the ph values of strong acids are lower than that of weak acids, which explains why the rate of reaction of strong acids with substances (such as.
Strong Acids Dissociate (Break Apart) Completely In Water).Strong acid passes electricity faster whereas weak acids are slow conductors. A weak acid is an acid that partially dissociates into its ions in an aqueous solution or water. In contrast, a strong acid fully dissociates into its ions in water.
Belum ada Komentar untuk "Strong Acids Vs Weak Acids"
Posting Komentar